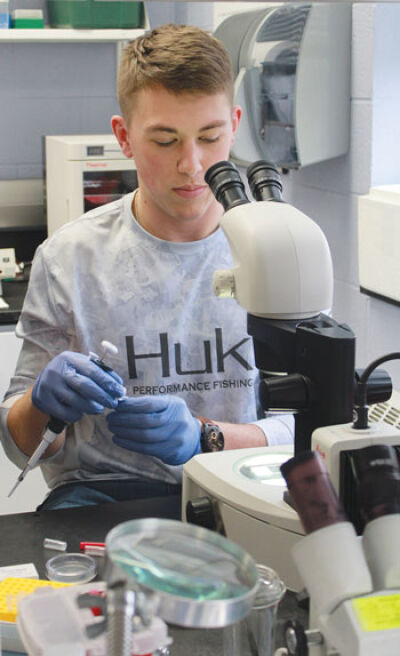
LTU undergraduate students will work with professors Shannon Timmons and Aleksandra Kuzmanov to create close chemical cousins of bisphenol A and test them on lab worms.
Photo provided by Matt Roush
SOUTHFIELD — On Feb. 23, Lawrence Technological University received a $446,867 grant from the National Institutes of Health to create close chemical cousins of bisphenol A (BPA). Shannon Timmons, the chair of the department of natural sciences and an associate professor of chemistry, and Aleksandra Kuzmanov, an assistant professor of biology, will lead the study.
BPA has been used to produce sturdy plastics since the 1950s. It is commonly found in everything from eyewear to food packaging to household products.
“This is a collaborative toxicology project where we’re investigating this Bisphenol-A. As I’m sure you may have heard of BPA-free products before, a lot of the public has heard of this. We were interested in BPA. It’s a known endocrine disruptor that has effects on human health and probably more effects than just endocrine disruption, but it has negative health effects on humans and the environment, so we are interested in finding a safer alternative.” Timmons said.
Kuzmanov explained that BPA messes up human hormones.
“Usually, it’s estrogen and testosterone reproductive hormones, so we know that it could affect our reproductive health,” she said. “Recent research shows that it not only messes up our hormones but also affects the quality of our reproductive cells, which means it could contribute to infertility. Many people are having trouble conceiving and keeping pregnancy. So one of the reasons could potentially be — and there is some evidence for it — BPA and similar cousins.”
Timmons’ undergraduate students will be working in the lab to create new bisphenol analogues, which are similar in structure to BPA. She explained that the BPA molecule is constructed of two six-membered rings of carbon atoms with various hydrogen and oxygen atoms attached. The students will work to change the atoms attached to the rings, using atoms such as bromine and fluorine to create novel bisphenol structures.
“A lot of times, with the BPA-free products, as a chemist, I wonder what is in them instead, because you can’t just take out a molecule from plastic and have it have the same physical properties, the same malleability, the same durability. All those kinds of physical properties that make plastic what they are, are based on the kind of chemicals used to make them,” Timmons explained.
“We did some investigations and found some journal articles that were published that showed that a lot of times, instead of BPA, manufacturers are replacing BPA with another bisphenol, a close chemical cousin, often BPS or BPF. These are just different bisphenols within this class of molecules. To the public, this seems good because they heard BPA is bad. So you hear BPA-free and think, great, but it’s actually not so great.”
According to Timmons, some alternatives used to replace BPA in consumer products are just as harmful or even more dangerous to human health because they have yet to be as rigorously tested. She explained that in this study, they will be taking a unique approach. Instead of changing the part of the molecule that most scientists have focused on altering and have not seen successful results with, her students will change a different part of the molecule to create new analogues that have never been made before. Timmons reported that studies have suggested that this might create a safer alternative.
The two professors have been collaborating on safer alternatives to BPA since 2018. Timmons altered molecules in a similar way to what other scientists were doing and found that it did not yield a safer alternative, which led to this new approach of creating brand-new analogues.
After Timmons’ students have created the new analogues, Kuzmanov’s students will test them on C. elegans worms.
“She’ll make a set of five or six different BPA cousins. And then I’ll be testing them with my students, also undergrad students, with worms, because worms make reproductive cells the same way we do. Basically, we’ll expose them to these chemicals. Then, we’ll look at the quality of these reproductive cells. Once we compare all these derivatives of BPA, if we see that something is safer, that it looks like it’s not affecting reproductive cells, we’ll test them in human cells that produce hormones, estrogen and testosterone in a petri dish,” Kuzmanov said.
The grant funds the project until January 2027. Timmons explained that there is a possibility that the two will continue to work on this study and similar studies beyond the three-year timeline.
Kuzmanov emphasized that the answer isn’t to rid the world of all plastic but rather to be more careful with plastic exposure.
“If you look at the medical equipment and everything they use in hospitals, it’s plastic. It made our lives easier. We need to be careful. We can’t really say, ‘Oh, we’re gonna eliminate plastics.’ You can’t. But you can minimize exposure,” she said.
Kuzmanov and Timmons recommend storing food in glass containers instead of plastic and avoiding heating plastic food containers or placing them in the dishwasher. Timmons warned against leaving plastic water bottles in the car on warm days. Kuzmanov suggested swapping out a plastic water bottle for steel or glass bottles.
For more information, visit LTU.edu.
 Publication select ▼
Publication select ▼

























